Detecting Tick-Borne Viruses in Wyoming: Better Testing, Better Protection
Better Testing, Better Protection
When ticks test negative for pathogens, how can we be sure the results aren’t compromised by degraded sample quality and technical errors? That question drove a recent project by our team to improve the diagnostic accuracy for tick-borne viruses—and it’s already making a difference for Teton County public health! Thank you to Kelsey Mitchell, Teton County Weed and Pest Biologist, and Mikenna Smith, Teton County Weed and Pest Entomologist, for conducting this research and in preparing this presentation that drives solutions for our community!
Teton County Weed & Pest Programs
Teton County Weed & Pest has 3 major programs:
- Vector Surveillance - Involves mosquito abatement and tick surveillance, including field work for dipping and trapping specimens, lab work for identifying vector species and testing for local pathogens and disease, and informing Teton County Public Health and the Wyoming Department of Health on our findings.
- Invasive Species Management - involves widescale herbicide spraying and Early Detection and Rapid Response solutions to manage noxious weed infestations that crowd out native plants across Teton County. We, also, provide property consults and small-scale solutions for local homeowners.
- Outreach and Education - involves informing the general public, tourists, and ranchers of the services we provide in mosquito abatement, tick surveillance, and weed management to help protect property, public health, pets and livestock. It involves going into the classroom, providing marketing materials and mailers to the community, and informing the public through PR and newspaper articles, blogs, social media, and newsletters of any items of public interest that come up in our research along with enrollment opportunities in community programs that our District provides.
TCWP Vector Surveillance Program - Why It Matters
Our vector surveillance program includes active & passive surveillance as well as pathogen testing. Ongoing vector surveillance is critical in supporting public health by informing the community on what vector-borne diseases are on the rise locally and how to protect yourself, family members, and pets. But testing for diseases isn’t foolproof—tick samples can degrade before they even reach the lab, leading to false negatives when putting them through pathogen testing.

TCWP Pathogen Testing Workflow
To understand the intricacies of testing protocols, it helps to have background on what our overall workflow looks like in the lab.
Our Tick-Borne Pathogen Workflow goes as follows:
1. Tick Collection:
Involves the collection of ticks and transport of samples back to our lab. Upon receipt, our team gives pre-quality control checks ensuring ticks are alive, not blood-fed, and verify the species and what life stage they are in. This work helps to ensure the sample is suitable for RNA/DNA extraction and pathogen testing.
What is DNA? What is RNA?
DNA stands for Deoxyribonucleic Acid, which is a very stable, double-stranded helix made of nucleotides (A, T, G, and C). It stores the genetic blueprint for any living organism.
RNA stands for ribonucleic acid. Is it an especially fragile single-stranded genetic material; the nucleotides are (A, U, G, and C - Uracil instead of Thymine). It is easily degraded by heat, light, or enzymes. It carries instructions from DNA to make proteins, or serves as the genetic material for some viruses.
2. Sample Processing / DNA Preparation
Involves the Sterilization of all surfaces to remove any contaminants, Homogenization which involves mechanically breaking the tick tissue, Inactivation - a dual chemical/heat treatment to kill any live pathogens for safe handling, and Aliquoting which divides homogenate for multiple tests if needed.
These steps are rough on RNA, which is highly sensitive. Degraded RNA can cause false negatives, the reason for our IPC testing protocol and this research. Our developed IPC (internal positive control) helps to check the sample quality during testing.
3. Nucleic Acid Extraction
This step involves extracting RNA (For RNA viruses) or DNA (for bacterial or DNA pathogens). This step ensures integrity and purity of the tick samples.
4. Reverse Transcription (for RNA viruses)
RNA viruses cannot be directly amplified by PCR. We convert viral RNA into complementary DNA (cDNA) using reverse transcriptase. This cDNA serves as the template for downstream PCR application.
What is PCR?
PCR stands for Polymerase Chain Reaction, and involves one of the most important processes we use in the lab. PCR is used to make millions of copies of a specific DNA sequence from a small sample. This amplification allows our team to detect tiny amounts of genetic material, which is crucial for testing ticks for viruses or bacteria.
PCR uses repeated cycles of heating and cooling to copy DNA. The steps: denaturation - heat separates the strands, annealing - cools the sample so primers bind to DNA, and extension - DNA polymerase builds a new DNA strand. These steps are repeated 25–40 times, doubling the DNA each cycle — leading to amplification and helps our team to identify pathogen presence in minute samples.
Standard PCR: Copies DNA and gives a visible band on a gel.
qPCR (Quantitative PCR / real-time PCR): Uses fluorescent probes to measure DNA quantity in real time, which allows our team to detect and quantify pathogens
5. PCR/qPCR Amplication
Reaction set up includes:
Pathogen - specific primers/probes
Setting an internal positive control (endogenous or exogenous)
Negative and positive controls for the assay
Detection: flurorescent signals measure the presence and quantity of the target.
Endogenous refers to a control that comes from the sample itself, exogenous refers to a control that is added from an external source.
6. Quality Control & Interpretation
Internal Positive Control (IPC): Confirms the sample has intact nucleic acid
(Negative controls: Detect contamination, Positive controls: Confirm the assay is functioning)
Cq/Ct values: Determine whether pathogen is present; failures may indicate poor sample quality or technical errors.
7. Data Analysis & Reporting
We compile results for each tick and generate results into our database and prepare reports on our findings to inform public health.
Developing The Internal Positive Control (IPC) for TCWP
We needed a way to ensure the sample RNA quality of every tick prior to pathogen testing because RNA (used to test for pathogens) is highly sensitive to temperature, light, freeze/thaws, etc, where DNA is more stable and can be stored for years. Because accurate pathogen testing relies on high quality nucleic acid, retaining RNA integrity is a major concern in our testing program.
We have a long list of tick-borne pathogens that we eventually want to test for, but the first two we are prioritizing are the tick-borne viral pathogens CTFV (Colorado Tick Fever Virus) and POWV (Powassan Virus).

For this research, I have focused on development of an IPC (Internal Positive Control, which is an essential component used to validate sample quality of each tick specimen) for use in our pathogen testing program. This will help our team to determine true negative test results from false negative test results, detect PCR (Polymerase Chain Reaction) inhibitors or technical errors, and improve overall assay reliability and, ultimately, confirm whether the pathogen test results are valid, due to ensuring high quality sample control for each tick tested.
Our Internal Positive Control, or Tick Sample Quality Control Process
- Every tick test includes taking a tiny snippet of a tick gene (calreticulin) that should always show up if the test is done right.
- If that gene doesn't show up, it signals that something went wrong—whether with the DNA sample, the reagents, or the process.
- This means:
- A “negative” result is reliable only when the IPC confirms the sample quality of the tick and the tick DNA sequence is properly amplified with the PCR machine
- We can catch errors before bad data gets reported
Ticks in our collection are only tested for pathogens IF:
- They are Rocky Mountain Wood Ticks, (Dermacentor andersoni)
- Not blood fed
- Alive upon receipt at the Teton County Weed and Pest Lab
Results + Supporting Research
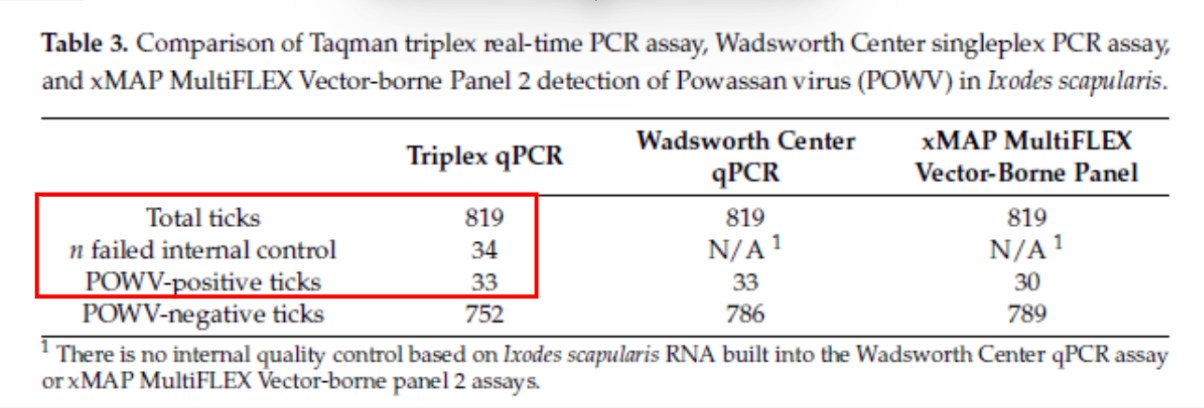
- We partnered with and adapted a method from researchers at UMass & NEVEC who found up to 4% of the ticks they processed failed to amplify the internal positive control or make many copies of a specific nucleic acid sequence.
- In our Wyoming tick samples for Dermacentor andersoni, the IPC (internal positive control used to determine tick viability) worked reliably—showing a strong signal.
- We confirmed that adding the IPC didn’t reduce our ability to detect CTFV-positive (Colorado Tick Fever-Positive) ticks—so both targets are compatible in the same test.

The characteristics of the IPC (Internal Positive Control) developed that I valued are:
- It’s an endogenous (produced internally) positive control
- Targets the calreticulin gene – multifunctional protein – in many cell types, which should be in high abundance
- For you Biology nerds: If the primers/probes spans an exon exon junction - it only amplifies RNA target, not genomic DNA; which is ensuring that it’s an appropriate control for viral RNA targets

Future Directions for Tick-Borne Virus Detection
I’m definitely excited about success of this testing as it increased our confidence in extraction protocol and continuous quality control of ticks by:
- Increasing our ability to train early career seasonal staff (and still ensure high quality data)
- Confirming that negative CTFV/POWV results = true negatives
- Detecting inhibition – identify PCR inhibitors and inform appropriate pool sizes

Impacts to Teton County
Improvements to our testing protocols locally leads to more reliable results for the community. Our public health and state wildlife agencies can trust negative results coming from our lab - and identify true outbreaks faster. New team members can follow clear protocols and still produce high-quality data and we can now roll out testing that includes other viruses (like POWV, Powassan Virus) and even bacteria like Tularemia (Which saw a large spike in Wyoming cases in 2024) - with confidence.
By adding a simple internal check and intervention, we’ve made our mosquito and tick virus surveillance program more robust, scalable, and trustworthy —right here in Jackson.
References:

%20(2).png)