Diversifying the Capacity of the District’s Lab to Provide Genetic Identification of Invasive Plants
Thank you to Kelsey Mitchell, Teton County Weed and Pest Biologist, for her contributions to this article, as part of her research presentation at the NAISMA Conference in 2024.
From "A Closet with a Microscope" to a Molecular Lab: Expanding TCWP's Capacity
Historically, the Teton County Weed and Pest Lab served primarily the vector surveillance side — the PEST side — of Weed & Pest.
A vital component of any effective vector surveillance program is, of course, species identification (microscopy). At one point, that was the lab’s primary function. As my colleague Mikenna Smith, Entomologist at TCWP, likes to say, the lab was once “a closet with a microscope.”
But over time, Mikenna transformed that closet into a functioning laboratory — evolving it to meet the growing needs of a modern vector surveillance program. This included adding qPCR-based pathogen testing and implementing a tick surveillance program.






Much to her delight, she helped grow the TCWP lab from “a closet with a microscope” to a fully equipped BSL-2 lab that follows BSL-3 practices — capable of handling almost anything you throw at it.
So, thanks to Mikenna’s hard work, when I joined the team, I got to just saunter in here and start playing.
Moral of the story:
- I’m in love with our lab.
- A lot of resources have gone into building the lab we have today. One of my goals has been to explore how we can effectively utilize the lab across more than one program.
We’re cruising on the Pest side… but how can we get the Weeds into the Weed & Pest lab?
Today, I want to share two projects that I have implemented to support growing the Weed portion of our casework, by introducing genetic identification of invasive weeds:
- One that I feel is a success
- And one that's ongoing
Project #1: Phragmites Genetic Identification
The first invasive species I’ve focused on for genetic identification is phragmites — or common reed — a perennial grass with several lineages. I’m focusing on differentiating the native and invasive subspecies of Phragmites australis.
The invasive subspecies forms dense stands, crowds out native vegetation (including its native counterpart), alters wetland hydrology, and increases fire risk.
While there are morphological differences between native and invasive forms, as Phragmites expert Kristin Saltonstall has noted:
“Due to the plasticity of the species and its ability to adapt to a wide range of conditions, it is difficult to distinguish definitively the native and introduced forms of Phragmites without genetic testing.”
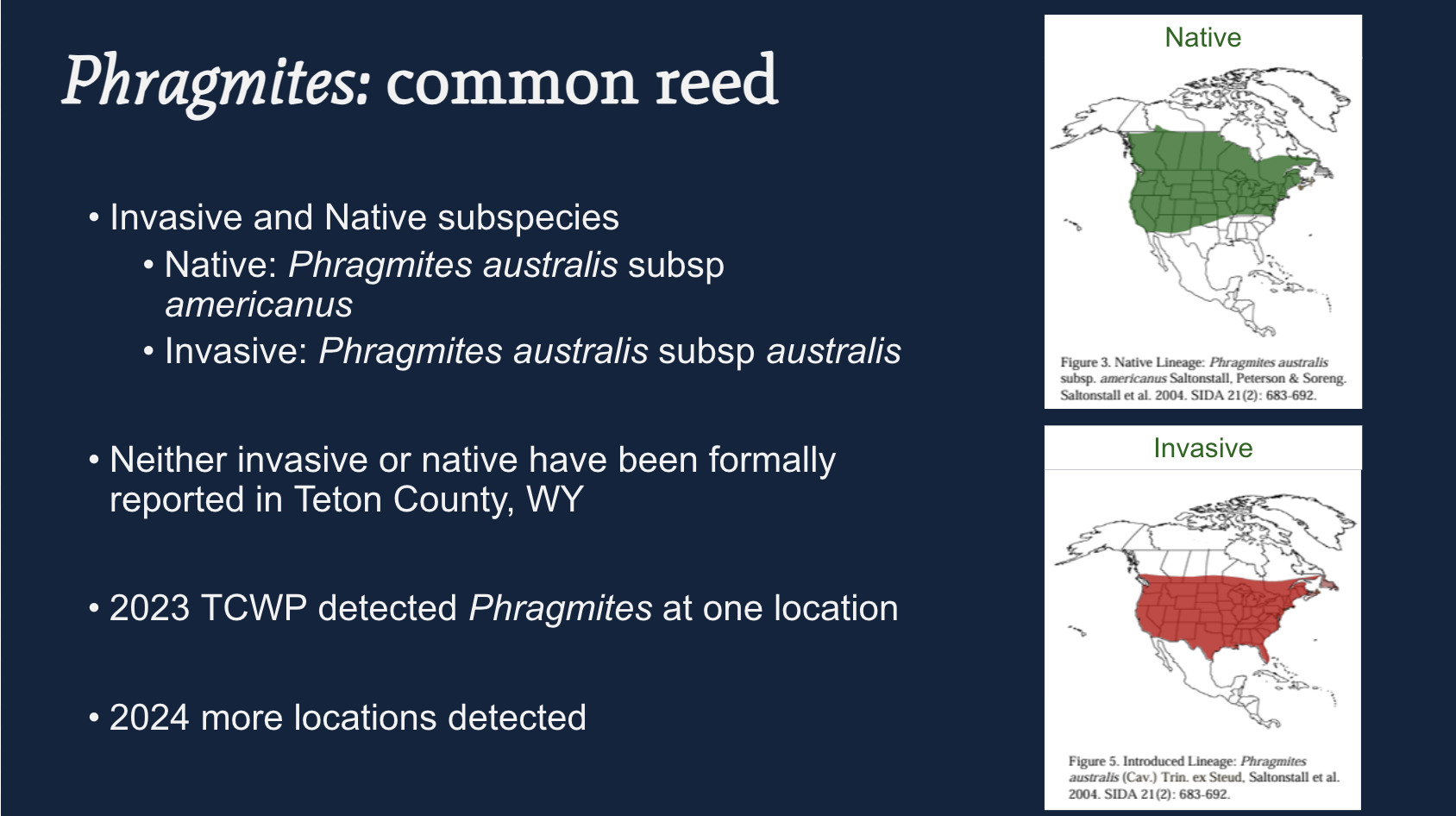
Context for Teton County
- Neither the native nor invasive subspecies had been formally reported in Teton County, WY.
- The native has historically been present in Wyoming (top map).
- The invasive has been reported in all lower 48 states, including counties surrounding Teton — but not Teton County… until recently.
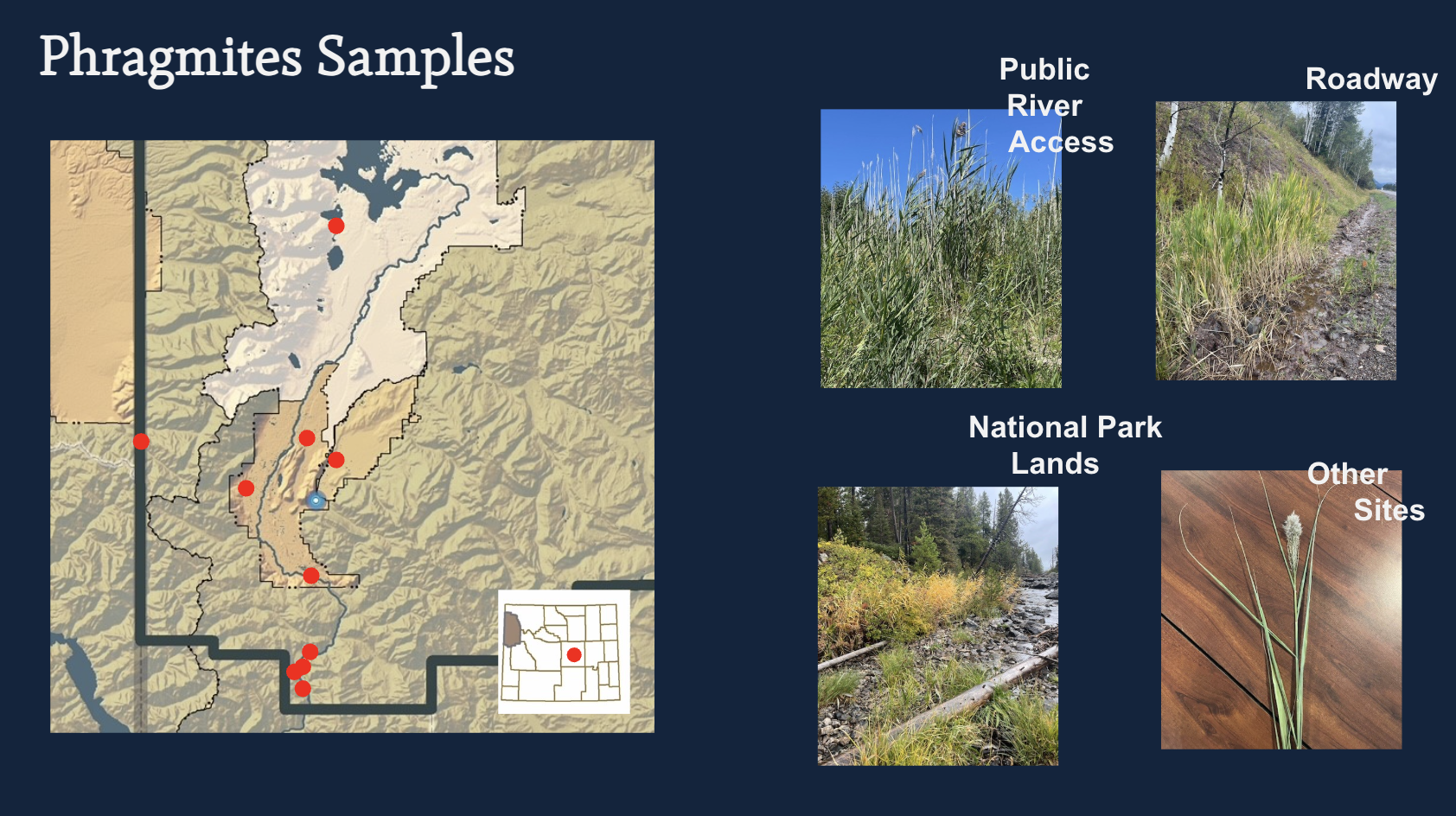
As of 2023, we found one sample in Teton County that appeared invasive based on location and morphology. This prompted us to begin molecular testing.

What We Did:
In 2023–2024, we collected 18 samples across 13 locations in Teton County.
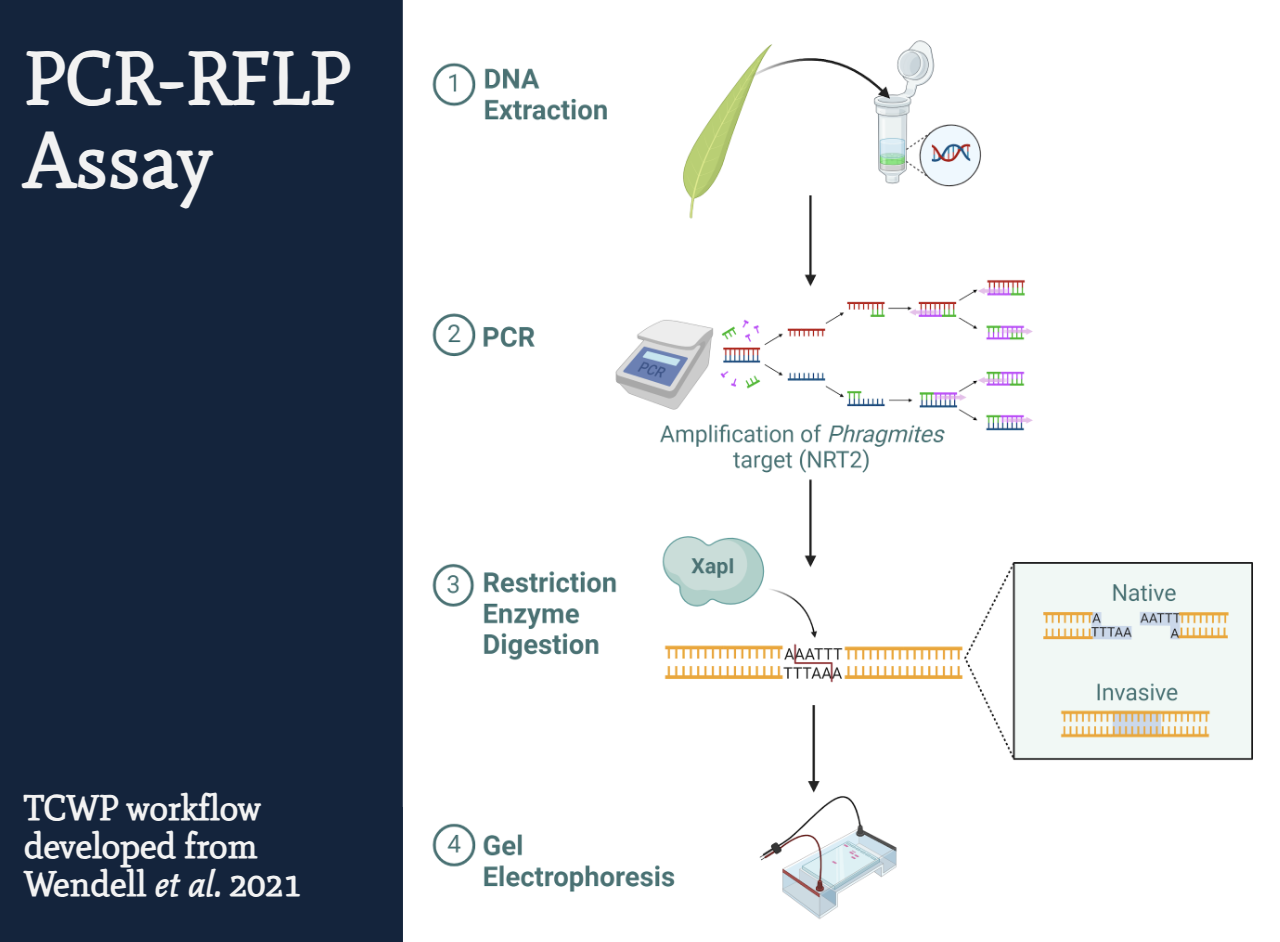
Genetic Identification Workflow:
- Extract DNA from the plant sample.
- Amplify a specific section of Phragmites DNA using PCR.
- Split the PCR product in half:
- One half is treated with restriction enzymes (REs) — molecular “scissors” that cut DNA at specific sequences.
- The other half is left untreated.
- REs will cut native DNA but not invasive DNA (due to sequence differences).
- Compare both halves using gel electrophoresis.
Each sample appears on the gel as two lanes:
- Native = two bands (cut)
- Invasive = one band (uncut)


Why This Assay?
In short:
- Quick to validate in-house
- Appropriate for small sample numbers
- Can detect hybrids
- Great training tool for seasonal lab staff
- No biohazard risk
Results:
- 3 sites = Native Phragmites
- 10 sites = Invasive Phragmites
- No hybrids detected
These are the first confirmed reports of both native and invasive Phragmites in Teton County. Validating and applying this test in-house allowed us to make informed management decisions quickly. We can now integrate this testing into our Early Detection Rapid Response (EDRR) program — ensuring that we remove invasive Phragmites while protecting native populations.
Project #2: Palmer Amaranth
Next up: Palmer amaranth, our next genetic ID target in the lab.

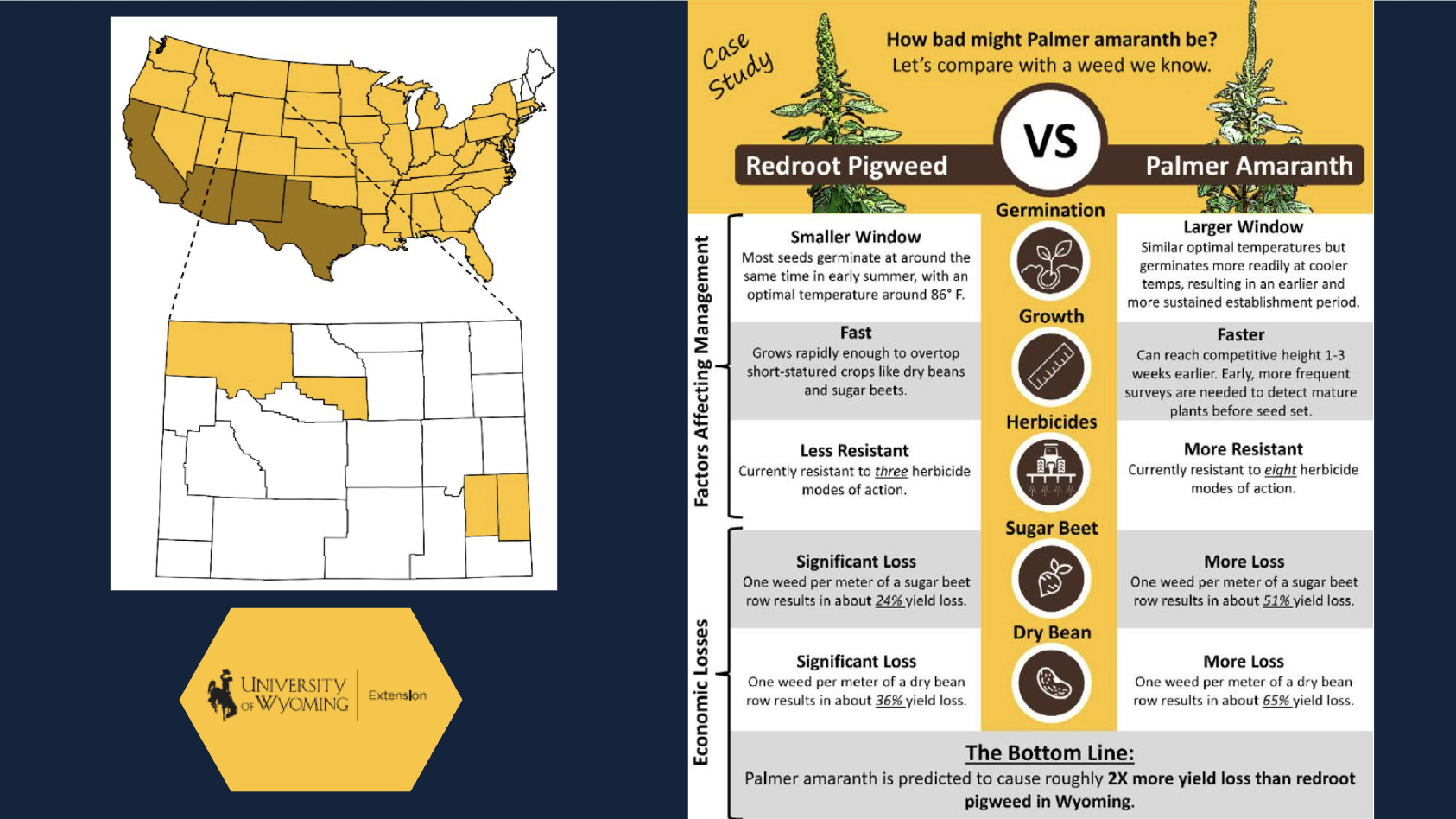
According to a bulletin from UW Extension (Aug 2024):
- Palmer amaranth is extremely difficult to control
- It’s resistant to 8 herbicide MOAs (Modes of Action)
- It causes significant economic losses — entire crop fields have been abandoned due to infestation
Challenges with Identification:
- Morphology is highly variable
- Seeds can't be identified without germination
- Palmer amaranth is a likely vector into the state
- Palmer amaranth has already reported in at least 4 Wyoming counties

Current Efforts:
We’re working to validate an in-house assay for genetic confirmation. But we’ve encountered a couple of challenges:
1. Assay Selection:
- Many published assays are available
- Some differentiate between 6 species — but not all relevant to our region
- Others require intensive time/reagents
- We're evaluating which assay fits our lab’s equipment and statewide needs
(Shoutout to Ben Walter, TCWP Lab Tech, who supported this work on validating primers and protocols.)
2. Positive Controls:
- It’s been a challenge to source genetically confirmed control samples for each target species
Looking Ahead:
We’re excited to continue expanding the capabilities of the Teton County Weed & Pest Lab — not just for vector surveillance, but also for invasive species management. Our work in genetic ID of invasive species is giving us the opportunity to give science-driven results that are guiding real-time decision making in the ongoing fight against invasive species on public and private lands.

References:

%20(3).png)